
Preimplantation Whole Genome Sequencing Test (PGT-WGS)
The Preimplantation Whole Genome Sequencing Test (PGT-WGS) allows for the examination of fertilized eggs for mutations that cause congenital diseases at the single nucleotide level before pregnancy. Furthermore, since fertilized eggs (embryos) can be cryopreserved, you can take your time to carefully consider which embryos to implant without worrying about time constraints.
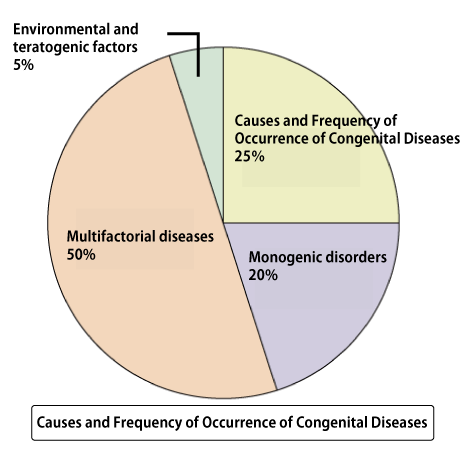
Approximately 3-5% of newborns are born with congenital diseases (congenital abnormalities). 25% of these cases are due to chromosomal abnormalities (such as trisomy, monosomy, translocation, etc.). Congenital diseases caused by a single gene account for around 20%, which is similar to the percentage caused by chromosomal abnormalities. Furthermore, congenital abnormalities caused by multiple factors (a combination of environmental and genetic mutations) reach up to 50%. Therefore, the proportion of congenital diseases caused by genetic mutations may be higher than those caused by chromosomal abnormalities.
Moreover, chromosomal abnormalities (such as trisomy or monosomy) become more frequent as age increases. Therefore, in younger individuals, there are reports suggesting that congenital diseases caused by genetic mutations may pose a higher risk compared to chromosomal abnormalities.
This Preimplantation Whole Genome Sequencing (PGT-WGS) test examines all genes at the level of base sequence in fertilized eggs before pregnancy, allowing the detection of mutations that may cause diseases, including newly occurring mutations, not only from parental inheritance. Based on the results of this test, it is possible to select embryos for pregnancy and perform thawed embryo transfer by returning cryopreserved embryos to the uterus.
Congenital Diseases Other Than Trisomy and Monosomy
It is known that 3-5% of newborns are born with congenital diseases.
25% of the causes are chromosomal abnormalities (such as trisomy, monosomy, translocation). Trisomy is a condition where one pair of chromosomes has three instead of the usual two. In humans, chromosomes usually come in pairs. However, in trisomy, there is an extra chromosome. Down syndrome is a well-known congenital disease caused by trisomy, where there are three copies of chromosome 21. Monosomy, on the other hand, is when there is only one chromosome instead of the usual pair. For example, Turner syndrome involves having only one X chromosome, which is a monosomy.
About half of the causes of congenital diseases are multifactorial diseases (a combination of genetic mutations and environmental factors). With advances in genome analysis, variations associated with diseases have been found even in diseases previously not thought to be caused by genetic mutations, such as lifestyle diseases. Therefore, many diseases are now considered to result from multifactorial (complex) inherited, where individual genetic mutations alone may not lead to the disease, but a combination of mutations does.
On the other hand, the proportion of congenital diseases caused by specific gene mutations is slightly less frequent at 20% compared to chromosomal abnormalities like trisomy. However, considering that multifactorial diseases are influenced by genetic mutations, congenital diseases caused by some form of genetic mutation may occur at a much higher frequency than chromosomal abnormalities.
In younger individuals, genetic diseases caused by mutations in single genes are more frequent
The frequency of numerical abnormalities in chromosomes is known to depend on the age of women. As women age, the proportion of eggs with numerical abnormalities in chromosomes increases. Therefore, as age increases, there is a higher likelihood of fertilized eggs or fetuses having conditions like trisomy or monosomy. Therefore, for women aged 35 and older, non-invasive prenatal testing (NIPT), chorionic villus sampling, and amniocentesis are recommended.
For younger individuals, the risk of numerical abnormalities in chromosomes (such as trisomy or monosomy) is not as high as in older individuals. Instead, there are studies suggesting that younger individuals have a higher frequency of congenital diseases caused by mutations in single genes compared to trisomy or monosomy.
Diseases caused by mutations in single genes may occasionally result in abnormalities in fetal morphology detectable by ultrasound examination. However, conventional tests like PGT-A (PGS), NIPT, chorionic villus sampling, and amniocentesis cannot detect mutations in single genes. As a result, abnormalities are mostly detected after birth.
Carriers of Genetic Diseases
The carrier screening is not perfect. As new genetic mutations causing diseases are discovered daily, carrier screening may not cover all genetic mutations that cause diseases. Additionally, spontaneous mutations can occur, leading to congenital abnormalities in children even when both parents have normal genes.
Although the probability of a spontaneous mutation is very small, at about 1×10-8 per base pair, the human genome consists of 3×109 base pairs. This means that approximately 30 new mutations occur per generation. Moreover, studies have shown that the likelihood of mutations in males increases with age, similar to the increase in chromosomal abnormalities in female eggs with age.
Not all of these mutations will cause congenital diseases, but depending on where the mutations occur, there is a possibility of developing congenital diseases. Naturally, these new mutations cannot be prevented by carrier screening or preimplantation genetic testing for monogenic disorders (PGT-M).
It is possible to detect mutations that may cause congenital diseases before pregnancy.
If there are chromosomal abnormalities, they can be detected by PGT-A (PGS). Additionally, if you are looking for the presence of mutations in specific genes based on family history of genetic diseases or carrier screening, they can be detected by PGT-M.
However, newly occurring mutations that cannot be detected through family history or carrier screening cannot be detected by PGT-A or PGT-M. In contrast, PGT-WGS examines all genes at the base level, making it possible to detect mutations that could cause congenital diseases, including such new mutations.
PGT-WGS Testing Method
In PGT-WGS, fertilized eggs are first created through in vitro fertilization (IVF). After about five days of culturing in vitro, the fertilized eggs undergo cell division and differentiate into cells that will become the future placenta and cells that will become the fetus. A portion of the cells destined to become the placenta is then sampled and used for PGT-WGS testing. Since it takes time to obtain the results of PGT-WGS, the fertilized eggs (embryos) are cryopreserved until the test results are available.
DNA is extracted from the sampled cells. This extracted DNA is then subjected to next-generation sequencing (NGS) to examine the entire genome's base sequence at a single-base level. The read base sequences are compared with the parents' base sequences to check for mutations that may cause genetic diseases.
The genome contains exons, which are the blueprints for proteins, and non-coding regions such as introns that connect them and do not serve as blueprints for proteins. Mutations in the exons, which are blueprints for proteins, often result in the inability to produce normal proteins. Although non-coding regions do not serve as blueprints for proteins, they include parts that control the amount, location, and timing of protein production. Therefore, mutations in non-coding regions can also lead to genetic diseases. This WGS examines the entire genome's base sequence, including non-coding regions, for any mutations.
Once the results of PGT-WGS are available the desired embryo for pregnancy is selected based on the test results, and frozen embryo transfer (FET) is performed to return the cryopreserved embryo to the uterus. Embryos can be cryopreserved almost indefinitely. Therefore, unlike prenatal tests such as NIPT, chorionic villus sampling, and amniocentesis, embryos can be considered for transfer without worrying about time constraints, allowing for satisfactory decision-making.
Limitations of PGT-WGS
However, PGT-WGS also has its limitations. Some mutations have unclear clinical impacts, and future research may change the interpretation of these mutations. Thus, mutations currently considered unrelated to diseases may later be found to be associated with diseases, or vice versa. Additionally, in cases of mosaicism, where only some cells in the body have mutations, there is a risk of missing these mutations, or mutations that cause diseases may be detected without the disease actually manifesting.
Furthermore, genetic diseases vary in penetrance and expressivity depending on the mutation, meaning that a detected mutation may not always lead to disease manifestation. NGS is not yet a perfected technology. While the accuracy of reading base sequences is very high, it is not 100%, so there is a possibility of missing mutations or incorrectly identifying potential disease-causing mutations.
Fee of PGT-WGS
Costs:
The total cost consists of the basic fee, the testing fee for embryos, and the WGS data storage fee.
- Basic Fee: 300,000 yen (excluding tax), 330,000 yen(tax included)
- Embryo Testing Fee: 250,000 yen (per embryo, excluding tax), 275,000 yen (per embryo, tax included)
- WGS Data Storage Fee:
- Couple's WGS: 23,000 yen per year (excluding tax), 25,300 yen per year (tax included)
- Embryo's WGS Data: 14,000 yen per sample per year (excluding tax), 15,400 yen per sample per year (tax included)
Inquiries │
We accept inquiries by email. Tokyo · Osaka │ O.G.M.S.

DNA Paternity Test for Aborted Fetus
english_help@ogms.biz
The email address may vary depending on the content of your inquiry. Please check the mail address on each service page.
There may be a delayed reply to inquiries received at night, weekends/holidays, winter and summer break.
It may take some time to reply depending on the nature of the inquiry.